UKCA | CE mark achieved
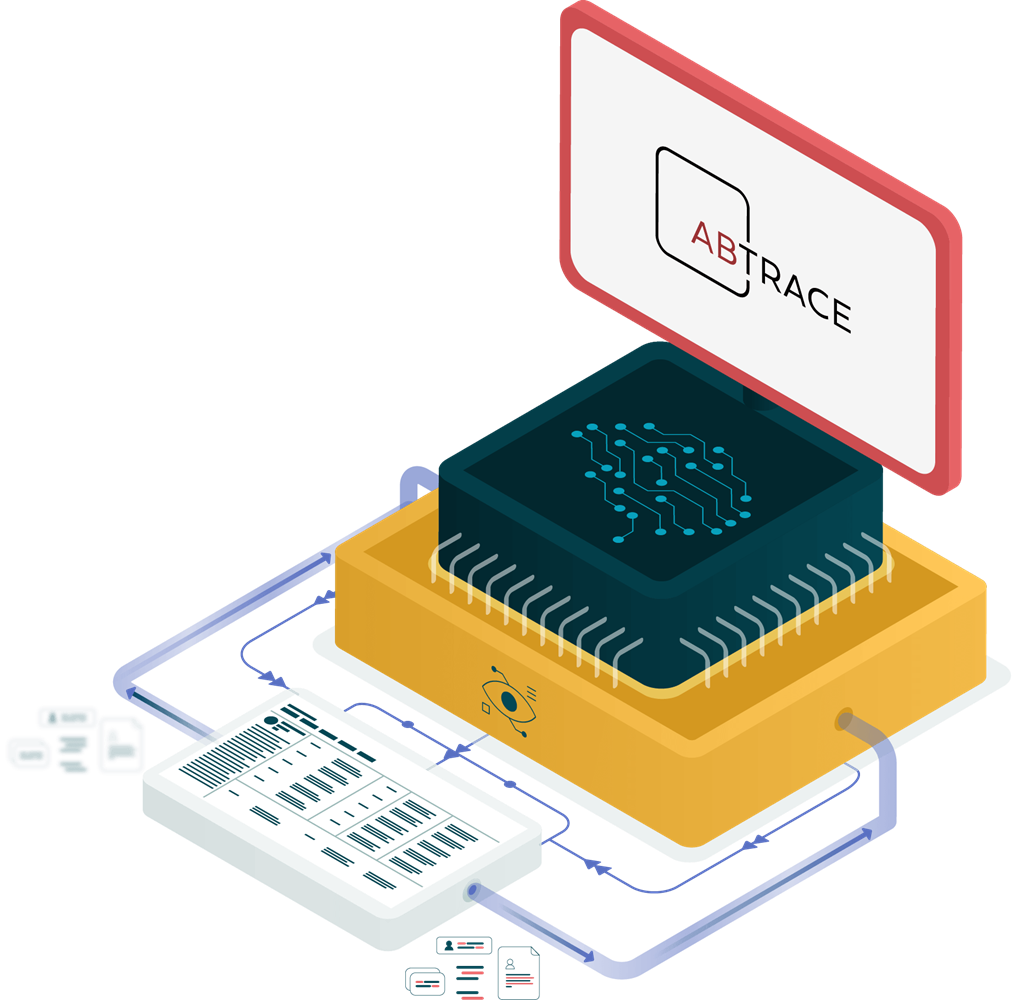
The UKCA and CE mark for a Software as a Medical Device (SaMD) confirms that the product undergoes verification and validation testing, risks are evaluated and mitigated, and that clinical evidence is collected. There is also an ongoing process for post marketing surveillance and quality assurance. This confirms that our clinical decision support software meets the standards for safety and performance for medical devices in the UK and EU, enabling its commercialisation in these markets.